In Which Compound Are the Bonds Best Characterized as Covalent
Here calcium acts as the cation with the carbonate species as the anion. Silicones are polymeric compounds containing among others the following types of covalent bonds.

Chemistry For Kids Chemical Bonding Chemistry For Kids Covalent Bonding Chemistry
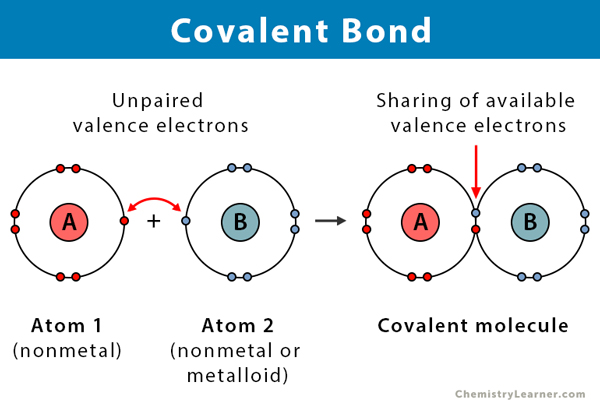
Covalent bond is a type of bond in which there is a sharing of electrons between the atoms involved.

. The compound that is classified as covalent bond is P₂O₅. The number of covalent bonds an atom can form is called the valence of the atom. E strong 35 As the number of covalent bonds between two atoms increases the distance between the atoms _____ and the strength of the bond between them _____.
As a rule of thumb metals often react with nonmetals to form ionic compounds or salts and nonmetals combine with other nonmetals to form covalent compounds. SiO SiC CH and CC. Covalent bonds can be best described as.
34 A _____ covalent bond between the same two atoms is the longest. However the carbon oxygen and nitrogen atoms can bond to more than one atom. However it can also be observed between nonmetals and metals.
Neutral atoms coming together to share electrons. Calcium carbonate is another example of a compound with both ionic and covalent bonds. You can determine the number of valence electrons for the light elements by counting the columns from the left.
Compounds formed by this type of bond are called covalent compounds. For example the hydrogen molecule H 2 contains a covalent bond between its two hydrogen atoms. The number of pairs of electrons in a covalent bond equals the bond order.
Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Nonpolar polar ionic metalic. Low Melting Point and Boiling Point.
Covalent bonds form when two nonmetallic atoms have the same or similar electronegativity values. Better source needed For many molecules the sharing of electrons allows each. Phosphorous P and chlorine Cl bond covalently to form the important industrial compound phosphorous trichloride.
A single B double C triple D they are all the same length. Starting on the far right we have two separate hydrogen atoms with a particular potential energy indicated by the red line. It is possible to define with a great deal of certainty the conditions under which anodic protection will work the best.
These species share an ionic bond while the carbon and oxygen. The electron pair donor is the ligand or Lewis base whereas the acceptor is the. This type of bonding occurs between two atoms of the same element or of elements close to each other in the periodic table.
Illustrates why this bond is formed. This bonding occurs primarily between nonmetals. We previously stated that the covalent bond in the hydrogen molecule H 2 has a certain length about 74 10 11 m.
The hydrogen atom and the halogen atoms form only one covalent bond to other atoms in stable neutral compounds. Acetylene has a triple bond a special. Bonds especially covalent bonds are often represented as lines between bonded atoms.
A covalent bond is a chemical bond that involves the sharing of electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. Covalent bonding does not result in the formation of new electrons. The absence of charged ions is the main reason behind this.
Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. Similarly each of the Cl atom gets 1 electron of Al to attain their stable electronic configuration of 8 electrons. This linkage is called a covalent bond.
Difference Between Ionic and Covalent Bond. Other covalent bonds also have known bond lengths which are dependent on both the identities of the atoms in the bond and whether the bonds are single double or triple bonds. Properties of Ionic vs.
The bond only pairs them. Covalent bonds are formed between non-metallic elements like hydrogen oxygen etc. Electrons are shared between two nonmetals.
Using the electronegativity values in Figure 46 arrange the bonds in order of increasing polarity and designate the positive and negative atoms using the symbols δ and δ. A covalent bond is formed between two similar electronegative non-metals. Some examples are HCl SO2 CO2 and CH4.
And this is the property of a. This unequal distribution of electrons is known as a polar covalent bond characterized by a partial positive charge on one atom and a partial negative charge on the other. Coordinate covalent bonds involve the unequal sharing of an electron pair by two atoms with both electrons originally coming from the same atom.
From the water. An exception to this is graphite where we see a cloud of electrons. Covalent bonding is the sharing of electrons between atoms.
Hydrogen and carbon are not bonded while in water there is a single bond between each hydrogen and oxygen. The correct answer to the question is Option C. The atom that attracts the electrons more strongly acquires the partial.
Bonds formed from covalent bonding have a Definite shape. Two electrons are shared between each hydrogen atom and the carbon atom bonded to it and four electrons are shared between the carbon atoms. The solid covalent compounds have soft structures like graphite.
Covalent bond exist between non metals only. When two dissimilar nonmetals form bonds eg hydrogen and oxygen they will form a covalent bond but the electrons will spend more time closer to. 1 lists the approximate bond lengths for some single covalent bonds.
A covalent bond is a pair of atoms that are stable and share electrons. Cl is a relatively large anion with a low charge density and is easily polarized by the hard cation giving the bond significant covalent character. Examples of important covalent bonds are peptide amide and disulfide bonds between amino acids and C-C C-O and C-N bonds within amino acids.
Ionic Bonds have No definite shape. The two fluorine atoms form a stable F 2 molecule by sharing two electrons. This is because of the presence of a cloud of electrons in between each layer of carbons atoms.
If atoms have similar electronegativities the. Covalent bonds include single double or triple bonds where 2 4 or 6 electrons are shared respectively. 7 rows Compounds containing covalent bonds are part and parcel of our day-to-day life.
This type of bond is formed between a metal and non-metal. When the atoms are approximately equal in their ability to draw electrons toward themselves the atoms share the pair of electrons more or less equally and the bond is covalent. These compounds are non-conductors of electrical charge.
So if two identical nonmetals eg two hydrogen atoms bond together they will form a pure covalent bond.

Covalent Compounds Covalent Bond Properties Examples With Videos

Covalent Bonds Biology For Majors I
Covalent Bond Definition Types And Examples

Ionic And Covalent Bonds Ionic And Covalent Bonds Covalent Bonding Electron Affinity
No comments for "In Which Compound Are the Bonds Best Characterized as Covalent"
Post a Comment